Pathology
Malignant melanoma (MM) comprises 3-4% of all malignant skin tumors nevertheless it is responsible for 60% of all fatal outcomes related to skin neoplasia. The incidence of malignant melanoma has significantly increased over the last 20 years among Caucasians worldwide. The highest incidence rates have been reported in Queensland, northern Australia. However, the incidence have been lower or at least stable amongst darkly pigmented individuals (Africans, Native Americans, Asians, Hispanics). Increased incidence is partly due to early detection of prognostically favorable melanomas (i.e., thin melanomas < 1.0 mm) and also partly due to a true increase in melanoma incidence. Recent decline claimed in some countries is, in part, due to the influx of low risk immigrants, diluting the true incidence of melanoma. With increasing life expectancy of population, melanoma appears to be a new public health problem and challenge in elderly patients.
Despite increased incidence of melanoma, the prognosis has improved due to earlier stage of presentation and detection and hence potentially curable stage.
Incidence of malignant melanoma is equal between men and women even though, based on some reports, it may be higher in women in some European countries. Melanoma is essentially a disease of adulthood (average 4-5th decade) and is uncommon in children. The anatomic locations of involvement in decreasing order are: Trunk (44%), Extremities (34%), Head & Neck (10%), and Acral (12%). In a typical scenario in male and female Caucasians patients it occurs in back and legs, respectively. In Africans, Asians and other dark-skinned individuals it is common on soles, mucosal sites, palms, subungual regions.
It is worth to mention the mnemonic of melanoma risk factors : M M R I S K
"K" _ Kindred (family history of melanoma)
Malignant Melanoma ABCD
Malignant melanoma ABCD is a simple but extremely important “must to know” that every health care giver should be familiar with since it may prevent a potentially curable atypical melanocytic proliferation from progressing to a disastrous malignant melanoma. These ABCD are abbreviation for: ASYMMETRY, BORDER irregularity (map like configuration), COLOR variegation (more than one color: light-dark brown color is usually due to presence of melanin in epidermis, blue and black colors due to melanin in dermis; red color due to inflammation and white color due to fibrosis in regression) and DIAMETER more than 0.6 cm. But as it is true in all disciplines in medicine many exceptions to this rule occur !
Melanoma Diagnosis
Any cutaneous lesion suspected for melanoma should be biopsied for histological examination, which remains the undisputed means of diagnosing melanoma. Due to the large number of melanoma mimics as well as the existence of borderline lesions, the histopathologic diagnosis of melanocytic lesions remains one of the most difficult areas in surgical pathology and dermatopathology in particular. Therefore, the examination of a suspected melanoma should always be performed by pathologists with specific experience of this type of cancer.
Excisional biopsy, whenever possible, should be preferred to incision biopsy, punch biopsy or shave biopsy, because in many instances they do not allow a complete and adequate evaluation of all histologic features and are inadequate for an accurate diagnosis.
Immunohistochemistry (IHC) is of great help in differentiating melanoma from other cancers and improves the detection of small metastatic deposits in the sentinel lymph node. Moreover, melanocytic differentiation markers (e.g. gp100, S100) and proliferative markers can support the diagnosis of melanoma in difficult cases.
Histological Classification
In 1967 malignant melanomas were originally classified by Dr. Wallace Clark and co-workers into several subtypes including. Superficial Spreading Melanoma (SSM), Nodular Malignant Melanoma (NMM) and Lentigo Malignant Melanoma (LMM). Later in 1976 Dr. Richard Reed added another subtype; i.e., Acral Lentiginous Malignant Melanoma (ALMM). Since then other less common and rare variants of malignant melanoma have been described as we will see later in this section. Distinctive histogenetic types led to the above subtyping concept, although some studies have shown any differences in overall prognosis is simply a matter of tumor thickness. Histological criteria for diagnosing malignant melanoma subtypes is basically is related to the location of the melanocytes (lentiginous, Pagetoid, etc) organization pattern of melanocytes (nested or single cells), cytological features, location of the malignant melanoma (for instance acral or subungual region) and other morphologic features such as lymphocytic host response.
Although histological diagnosis of melanomas remains subjective, it is still fairly reproducible amongst pathologists and dermatopathologists for more conventional subtypes.
Incidence of major subtypes of malignant melanomas is as follow:
- Superficial Spreading malignant Melanoma (SSM): 50-75%
- Nodular Malignant Melanoma (NM): 15-35%
- Lentigo Maligna Melanoma (LMM): 5-15%
- Acral Lentiginous malignant Melanoma (ALM): 5-10%
A novel classification of malignant melanomas categorizes them into:
1) Conventional melanoma with further sub classification based on the presence or absence of intraepidermal component as follows:
- Intraepithelial component: Pagetoid, Lentiginous, Nested, Mixed:
- Intraepithelial melanoma (Melanoma in-situ)
- Intraepithelial melanoma with invasion into dermis, subcutis, etc
- Absence of intraepidermal component
2) Conventional melanoma sub classified based on because of etiology and locations: Solar melanomas, Acral melanomas, Mucosal melanomas
3) Unusual and rare variants including:
- Desmoplastic/Neurotropic melanoma
- melanoma resembling nevi (for instance Nevoid Melanoma, Spitzoid melanomas)
- melanoma arising in or resembling blue nevi (malignant blue nevi)
- melanoma arising in a dermal or congenital nevus
- animal type melanoma (pigment-synthesizing melanomas, also known as equine-type melanomas)
- miscellany: Verrucous, Pleomorphic, Small cell, Balloon cell, Signet ring cell, Myxoid, Rhabdoid melanomas based on their morphologic cytology
In this proposed classification amelanotic melanoma is considered a poorly differentiated melanoma, usually of nodular type, rather than a special type.
Superficial Spreading Melanoma (SSM):
- De novo (75%)
- In association with preexisting nevi (Congenital and dysplastic) (25%)
- Any age ; any location
- Common on trunk in males
- Common in lower extremities in females
- Variation in pigmentation
- Irregular border
Superficial spreading melanoma (SSM) is the most frequent histological type of melanoma (about 65% of all cases).
It is characterized by the presence of a radial growth phase mostly composed of large epitheliod melanocytes haphazardly distributed at all levels of the epidermis in a pagetoid fashion.
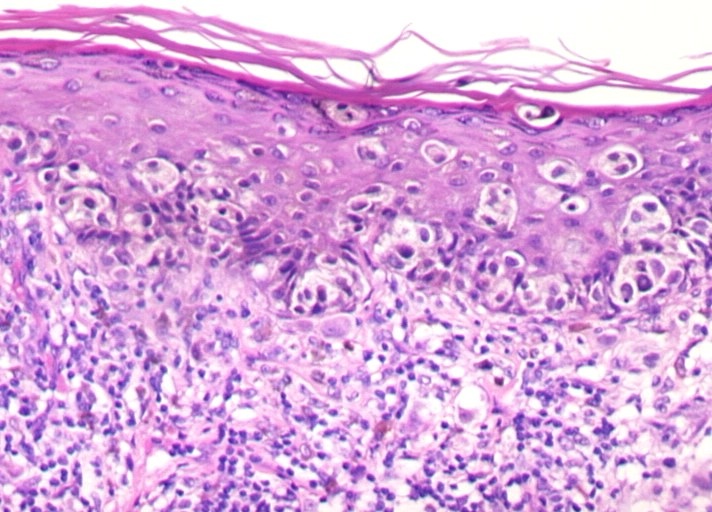
Nodular Malignant Melanoma (NMM):
- Anywhere in the body
- No antecedent RGP
- Nodular, polypoid, and sometimes pedunculated
- Dark brown to blue-black
- Occasionally, flesh-colored amelanotic
- Sometimes with ulceration
Nodular melanoma (NM) is the second most frequent histological type of melanoma (10 to 15% of all melanomas in Caucasians).
Unlike the other three main histological types, NM only has a vertical growth phase (the absence of a radial growth phase is the pathological landmark of this type of melanoma).
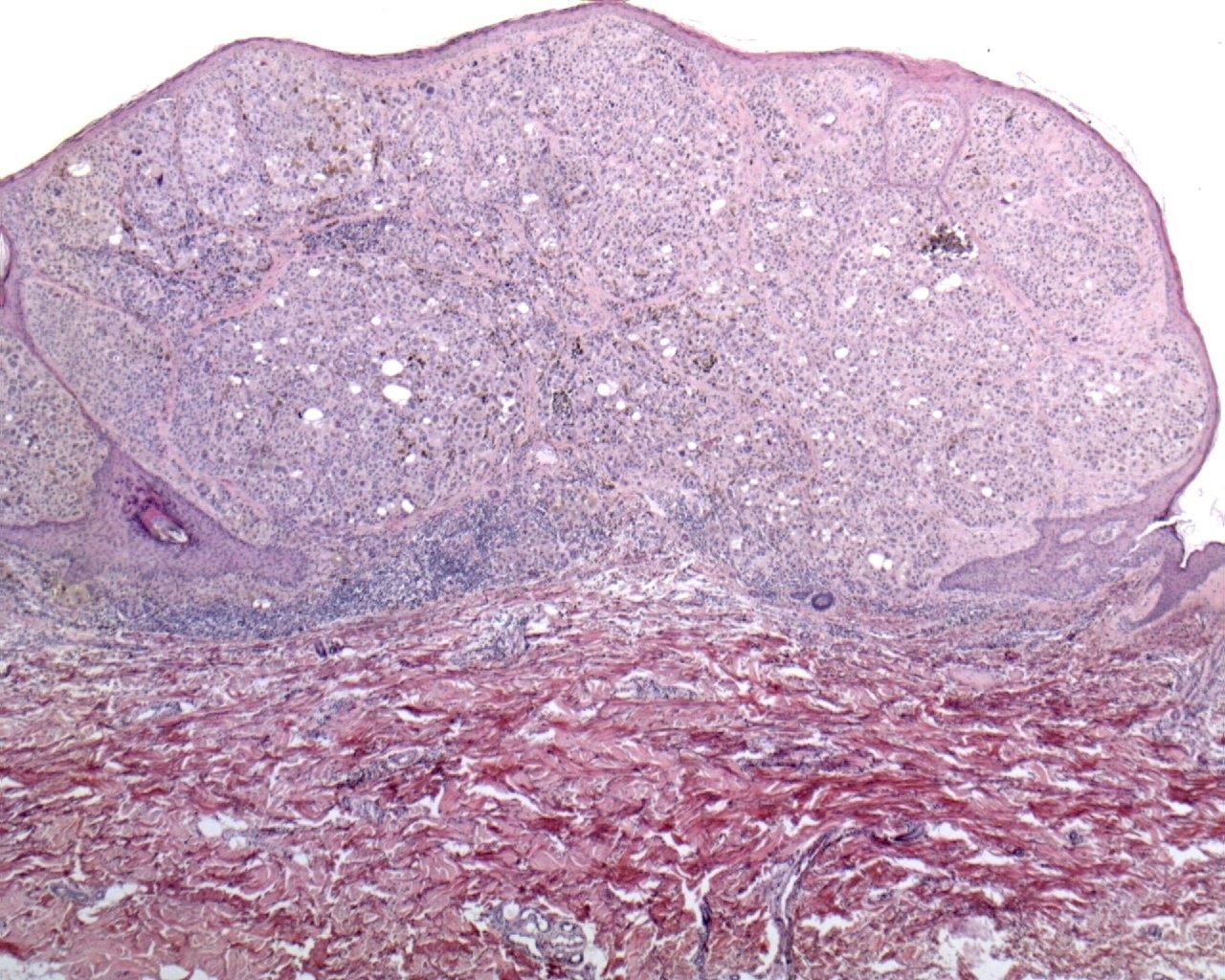
Acral Lentiginous Melanoma (ALM):
- Common in black people and Japanese
- Usually elderly ; male>female
- Palm, sole, and subungual skin
- Brown to black discoloration of the nail with breadth of pigmentation >3 mm
- Pigmented plaques or nodules with ulceration
- Extension to proximal nail fold (Hutchinson’s sign)
- Not all MM in this regions are ALM type
Acral lentiginous melanoma (ALM) is a relatively rare type of melanoma (about 5% of all cases). It arises in the non-hairy skin of the palms of the hands, the soles of the feet and the nailbed (subungual melanoma).
The clinical picture may be variable due to the presence of thick skin (or nail) at these sites. ALM occurs rarely in whites, but encompasses 35% of the melanomas that develop in black races, Hispanics, or Asians.
The dismal prognosis of this variant of melanoma has been explained by its location at acral glabrous sites; a delayed diagnosis may contribute to worse prognosis. The radial growth phase of ALM is histologically characterized by a lentiginous proliferation of atypical melanocytes along the basal layer of the epidermis, and is associated with marked acantosis of the epidermis and elongation of the rete ridges. The vertical growth phase often presents a spindle cell component.
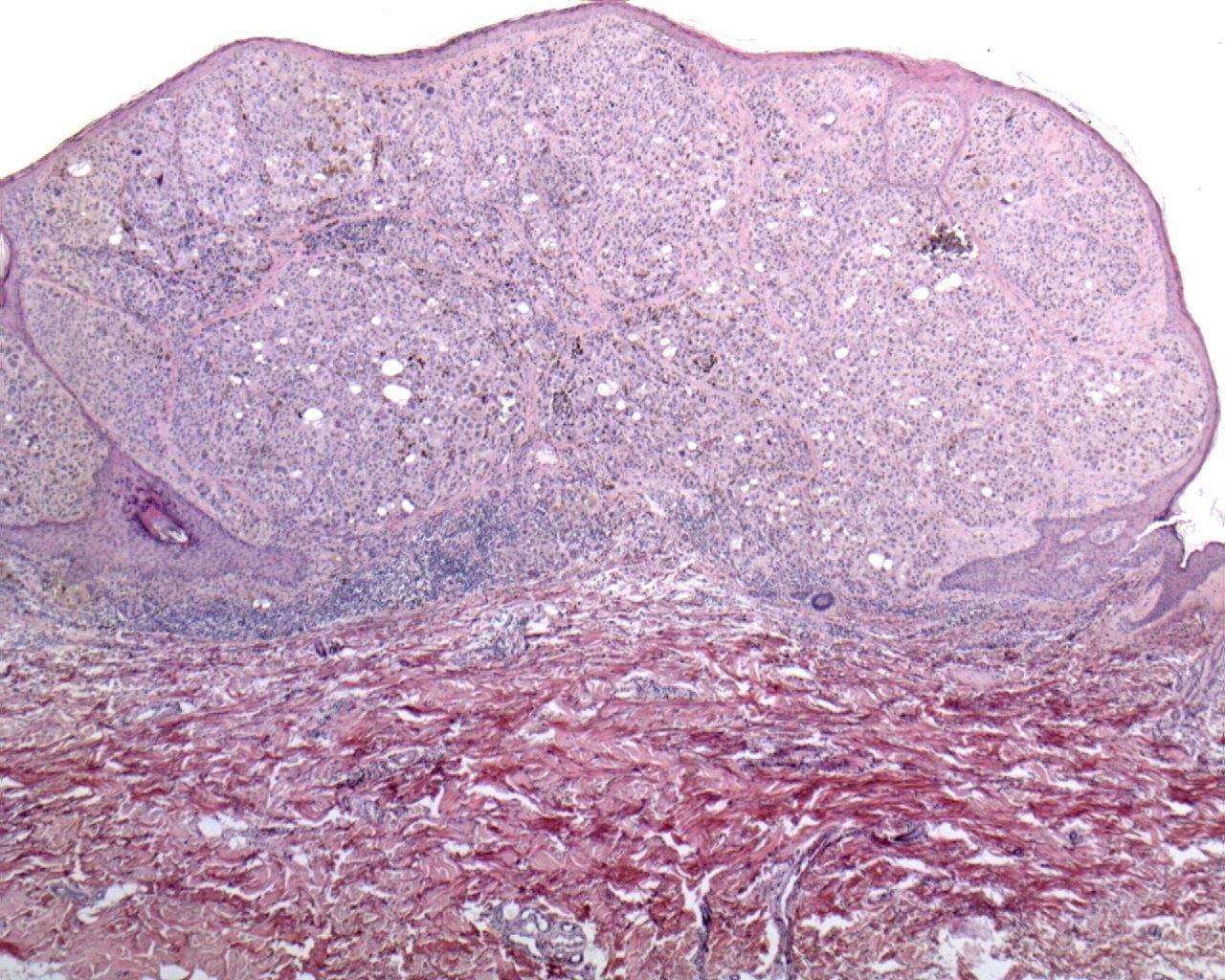
Lentigo maligna melanoma (LMM):
Lentigo maligna melanoma (LMM) typically occurs in older people, on sun-exposed areas (mostly head and neck).
LMM was considered to have a better prognosis than the other forms of melanoma, but recent studies have suggested that the different biologic behavior may be related to an inferior thickness at the time of diagnosis.
This variant of melanoma is histologically characterized by a confluent growth of atypical melanocytes along the dermal-epidermal junction frequently extending downwards the cutaneous appendages. The invasive component may be composed of spindle cells or may be associated to a desmoplastic reaction.
Rare Variants
- Desmoplastic Melanoma
- Nevoid Melanoma
- Verrucous Architecture
- Small Cell Melanoma
- Signet Ring Melanoma
- Myxoid Melanoma
- Osteogenic Melanoma
- Animal (Equine) Type Melanoma/Pigment Synthesizing Melanoma/ Macrophagic Melanoma
- Childhood Malignant Melanoma ex Congenital Nevus
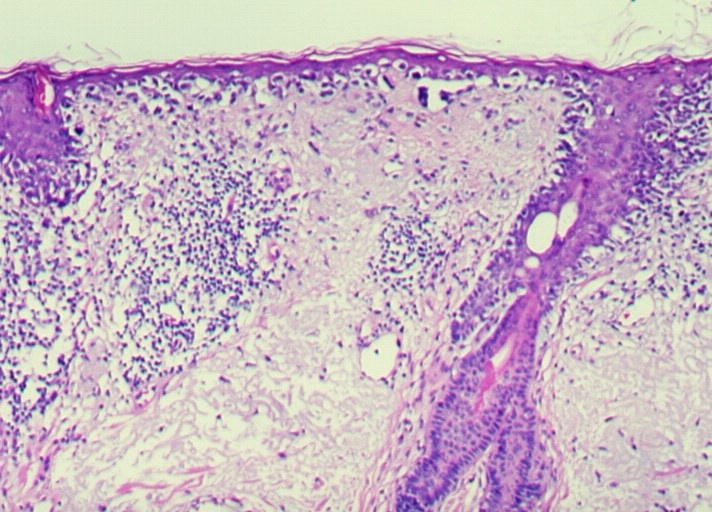
Desmoplastic melanoma
Desmoplastic melanoma (DM) is a rare variant of malignant melanoma (2% to 4% of all melanomas) that occurs predominantly on the head and neck of elderly patients. Due to the lack of characteristic features, DM is prone to be misdiagnosed both clinically and pathologically, so that the appropriate treatment is frequently delayed.
Indeed, DM shows a wide spectrum of morphological appearances from hypocellular variants to high grade lesions resembling different types of sarcomas. Immunohistochemistry has an important role in the recognition of this melanoma variant. The spindle cells are strongly positive for S100 and vimentin, while gp100 and the other melanocytes differentiation markers are negative or show a weak focal positivity.
Prognostic Factors
Prognostic factors in malignant melanoma can be classified in three groups:
A) Morphological (histology) factors
B) Clinical Factors
C) Other Factors
Histological factors with adverse effect :
- Breslow thickness
- Clark level
- Ulceration
- Mitotic rate
- Microscopic satellites
- Regression
- Lymphovascular invasion & Angiotropism
- Tumor volume
- Neurotropism
- Non-brisk Tumor Infiltrating Lymphocytes (TIL)
- Cell Type (spindle cells have better prognosis than the other cell types)
- Histogenetic type
- Vertical Growth Phase
Clinical factors with adverse effect:
- Advanced clinical Stage
- Worse prognosis with increasing age
- Men have worse prognosis
- Local recurrence
- Anatomic location (Head and Neck ,Palms and Soles have worse prognosis)
Other Factors with adverse effect:
- Increased nuclear volume
- DNA content
- Nucleolar Organizer Regions (worse with increasing AgNORa)
- Proliferation and tumor cell motility indices
- Circulating melanoma cells (RT-PCR or Immunohistochemistry)
Note: There are conflicting reports regarding pregnancy as an adverse prognostic factor. It seems that pregnancy has no effect on patients who have had a melanoma diagnosed and treated prior to pregnancy.
Breslow (Tumor) Thickness
- The most important single predictor of survival
- Quantitative measurement of tumor invasion
- From the most superficial aspect of granular layer to the deepest point of tumor
- If the adventitial dermal invasion is the only site of dermal invasion: measure from the inner aspect of the outer root sheath epithelium of the hair follicle
- If there is ulceration; measure from the base of the ulcer
Clark Level (Anatomic Level)
- Level I: Confined to epidermis
- Level II: Partial infiltration of the papillary dermis by single cells or small nests
- Level III: Tumor cells fill and expand the papillary dermis and come in contact with papillary-reticular dermal interface
- Level IV: Infiltration into the reticular dermis
- Level V: Infiltration into the subcutaneous fat
Radial & Vertical Growth Phases
The concept of Radial Growth Phase (RGP) and Vertical Growth Phase (VGP) was first introduced by Dr. Clark and colleagues and currently is widely accepted. Majority of malignant melanoma, except for some variants including nodular malignant melanoma, begin as intra-epidermal proliferation of malignant melanocytes (RGP). RGP sometimes remains confined for decades. RGP is almost always curable by surgical excision. Later, expansile nodules or aggressive infiltration of reticular dermis or subcutaneous fat (VGP) occurs. VGP is defined as dermal nests or expansile dermal nodule(s) composed of fully transformed malignant cells which exceeds the size of any junctional nest(s); i.e., tumorigenic and/or mitotic activity even if dermal nests are smaller than any junctional nests (mitogenic). The importance of VGP is that when a melanoma enters VGP it acquires the capacity to metastasize, in which the prognosis is directly related to depth of invasion.
In summary, three different clinical and histomorphological steps in tumor progression of malignant melanoma are as follows:
1. RGP-confined (confined to basement membrane- melanoma-in-situ)
2. RGP-confined, microinvasive (dermal nests are smaller than junctional nests and there is no mitosis present in dermis)
3. Vertical Growth Phase (VGP)
Note: Presence of large nest in Clark’s level II implies an incipient VGP. And involvement of Clark’s level III usually implies VGP. Guerry et al. in 1993 showed that 161 patients with RGP-confined without regression had median of 13.7 yr metastasis free survival. In another study by Taran & Heenan in 2001, only 5 of 1716 patients with Clark’s level 2 (with <1 mm thickness) developed metastatic disease; and all those five patients had established regression. Their conclusion from that study was that metastasis from Clark’s level 2 malignant melanoma occur rarely, if at all, in the absence of regression.
Cell Types in VGP
Common cell types are epithelioid and spindle cells, although mixed cells may also be seen. In general, spindle cells are associated with better prognosis than other cell types.
Ulceration
Ulceration in melanoma is defined by Interruption of the surface epithelium by tumor cells. When ulceration is present the Breslow thickness is measured from the base of the ulcer down to the deepest part of invasion. This is in contrary to Breslow thickness in nun-ulcerated melanomas in which the tumor thickness is measured from granular layer to the deepest part of the invasion.
Mitotic Index
Mitotic is basically the mitotic count per square millimeter. Based on the optical instrument used, each square millimeter is equal to 3-10 HPF. Various studies have shown that increased mitotic index is associated with worse prognosis, particularly if there are more than 6 per square millimeter.
Microscopic satellite(s)
Microscopic satellites are defined as dermal or subcuticualr nodule(s), clearly separated from and not contiguous with the main VGP. Presence of microscopic satellites have been shown to be associated with decreased incidence of disease-free survival and overall survival (Gernhard et al., 1998)
Regression
Complete regression signifies a worse prognosis. Complete regression is defined by absence of melanocytic proliferation in atrophic and effaced epidermis. Subjacent dermis shows nonlaminated fibrosis (and hence clinically looks white) with few inflammatory cells ands melanophages. One or both sides of this area still show malignant melanoma. Byers and Bahwan in 1998 concluded from their study that regression of 75% of tumor is the critical volume of regression that portends metastasis.
Angiovascular Invasion
Vascular invasion did not emerge as a significant variable in most early series of melanoma as it is uncommon; therefore, a large patient volume is necessary to uncover statistical evidence of prognostic significance. However, at least in some studies (such as the one from Mraz-Gernhard et al., 1998) has been shown to be a predictor of metastasis
Tumor Infiltrating Lymphocytes (TILs)
It appears that host response and TILs has a favorable prognostic value, although some investigators have found no association.
- Meta-analysis by international collaboration of investigators with retrospective study of primary cutaneous melanoma tissue is in progress to determine whether presence of TIL population is an important prognostic factor or not. At the time being malignant melanoma are reported as having Brisk, non-brisk or absent lymphocytic host response (TIL).
- Brisk or diffuse response is defined as lymphocytes throughout the tumor and/or along virtually the entire base of the tumor.
- Non brisk or focal is defined as only foci of infiltration less than the whole of the tumor and/or less than virtually the entire base of the tumor
- Absent TIL is defined by “no lymphocytes present”
Note: The important factor in TILs is the definition of TIL. TIL is defined as direct interaction of the lymphocytes and melanoma cells. If the lymphocytes are present but don’t have contact with tumor cells then this is considered “absent TILs”.
Precursor Lesion
Malignant melanomas may arise in association with pre-existing melanocytic proliferation including dysplastic nevi (usually those with high grade cellular and architectural atypia), Familial Dysplastic Nevi, Congenital Nevi.