Prognostic factors
[11] Molecular markers
Tumor thickness (T)
Together with nodal involvement and distant metastasis, tumor thickness is one of the most reliable prognostic predictors currently available (see TNM staging section) [1, 2].
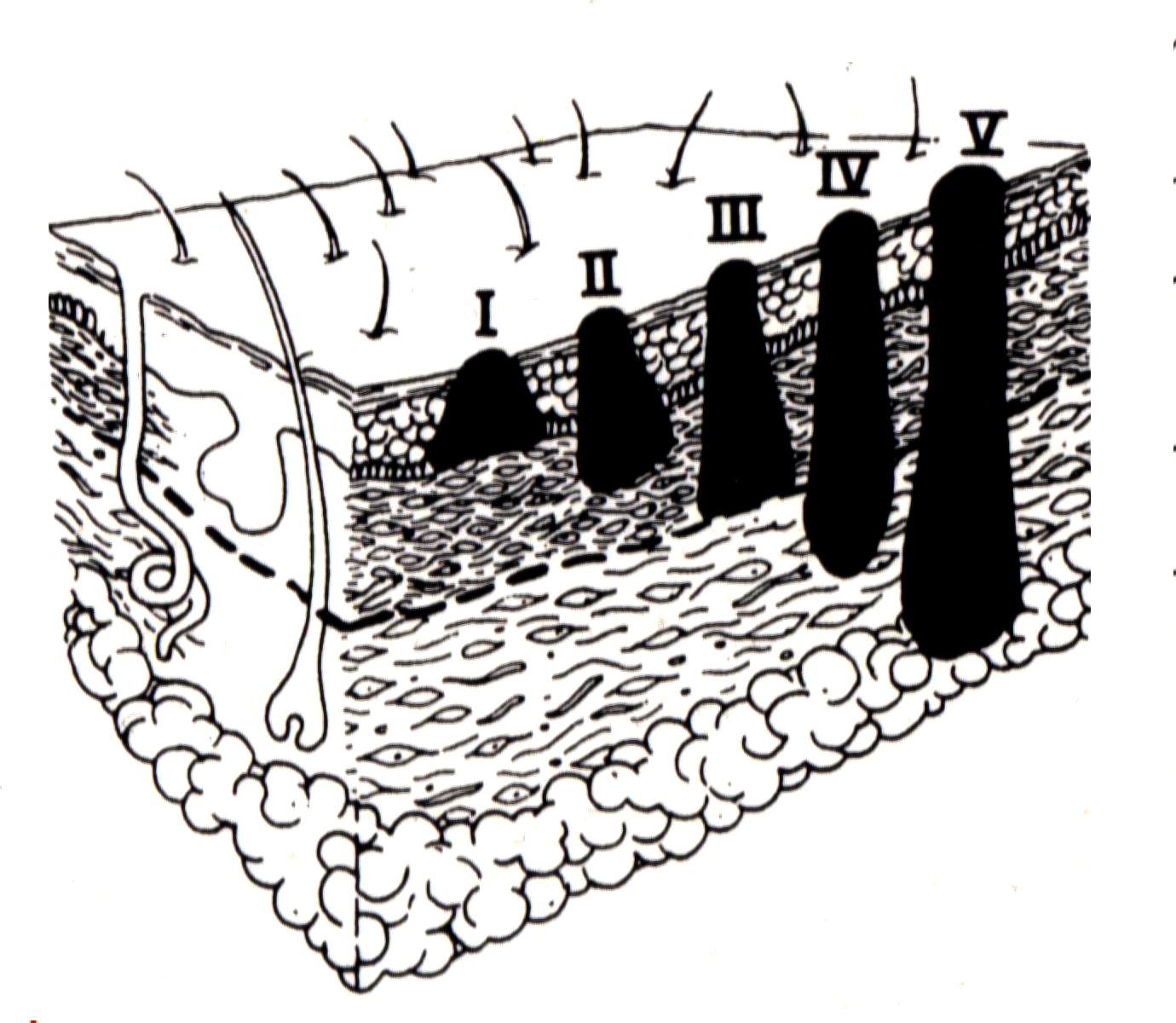
Two grading systems have been classically employed: 1) the Breslow's system is based upon the measurement of the maximum absolute thickness of the melanoma from the granular layer of the epidermis to the deepest point of invasion into the dermis: this is the most widely accepted system to grade melanoma depth and is adopted by the TNM staging system (see TNM staging section); 2) the Clark's levels describe melanoma depth according to the invasion of the four skin layers.
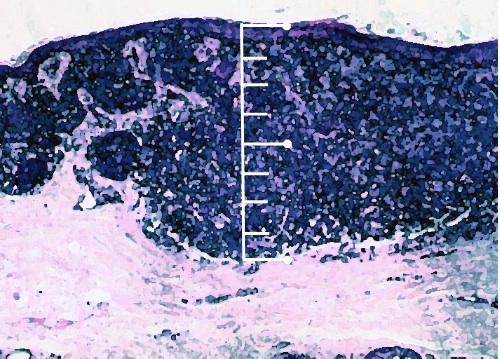
As it independently predicts disease recurrence and worse overall survival, ulceration has been implemented in the TNM staging system (see TNM staging section) [1, 2].
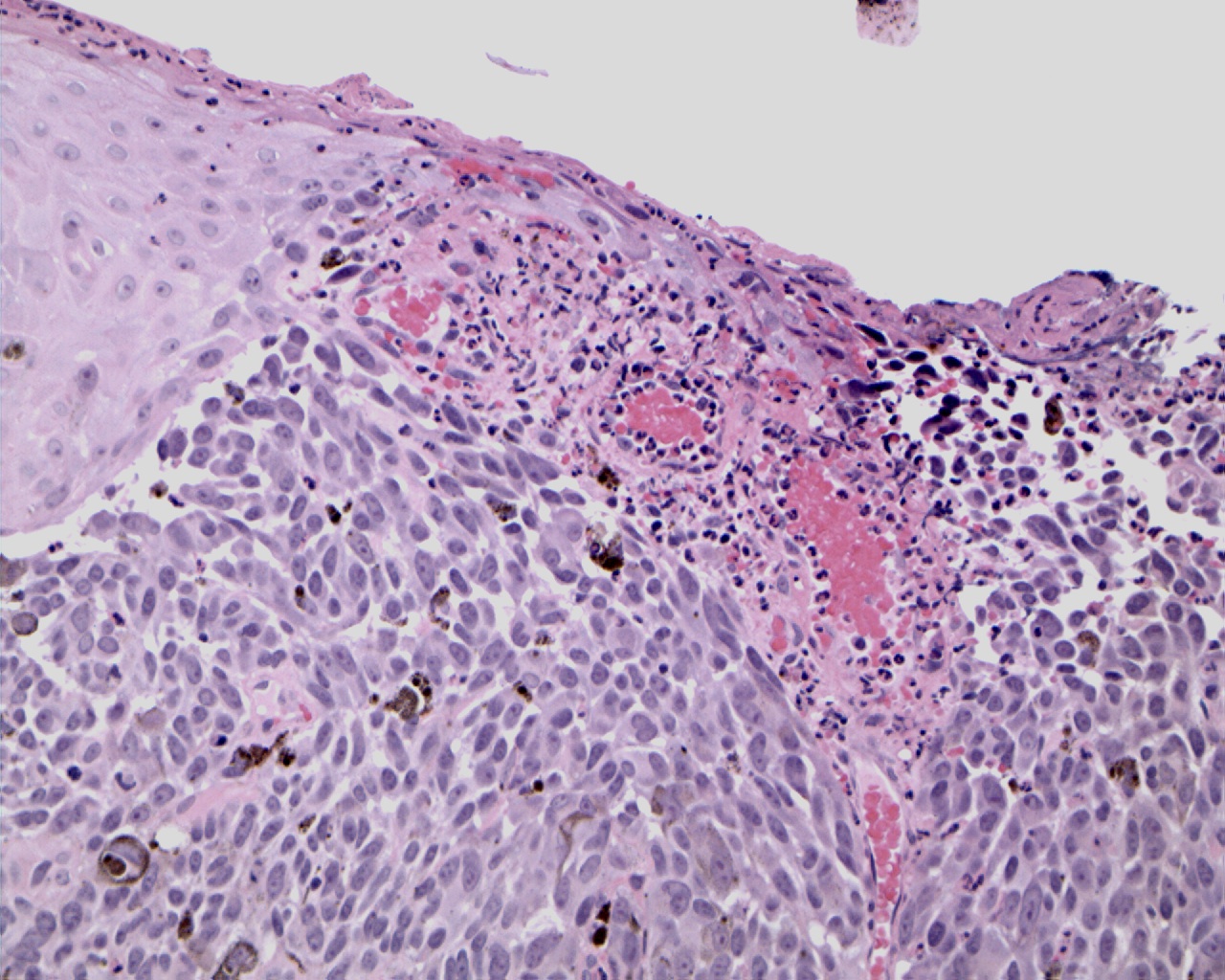
Regression is characterized by the replacement of tumor cells with lymphocytic infiltrate associated with variable dermal fibrosis, vascular proliferation and melanin-containing macrophages [7]. Its prognostic significance is still controversial. It has been hypothesized that the unfavorable influence may be related to a permissive role played by regression in the development of a vertical phase; on the other hand, regression may lead to underestimate Breslow depth, which ultimately causes the understaging of patients.
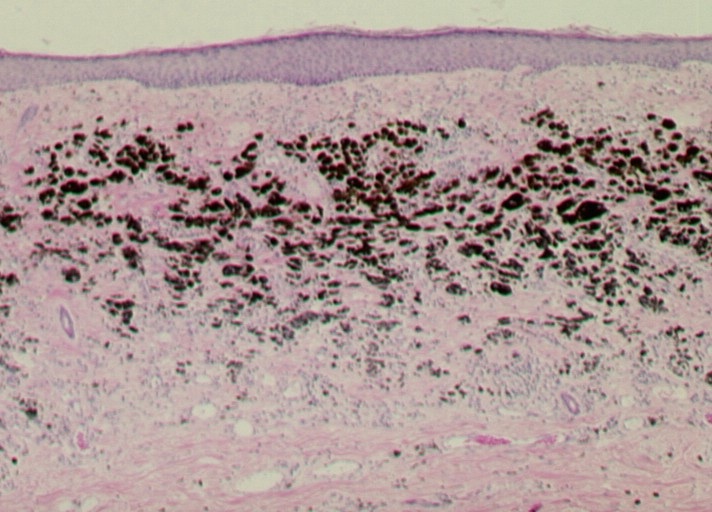
Mitotic rate is usually expressed as the number of mitoses per squared millimeter. Although its prognostic value is not universally recognized, several recent studies have demonstrated that the mitotic rate is an independent prognostic factor in localized melanoma [3-5].
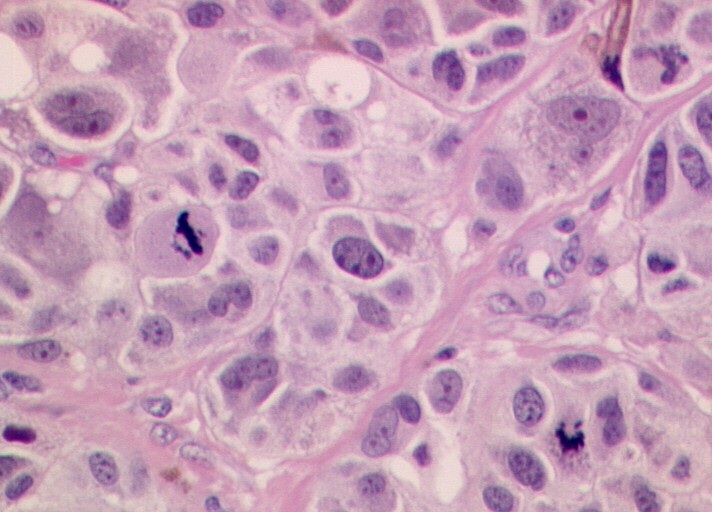
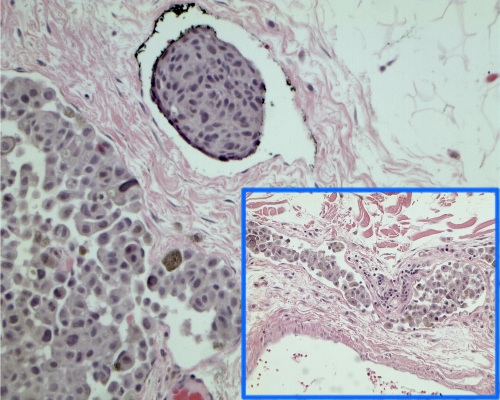
As for other solid tumors, the infiltration of blood and lymphatic vessels by melanoma cells has been associated with worse prognosis [6].
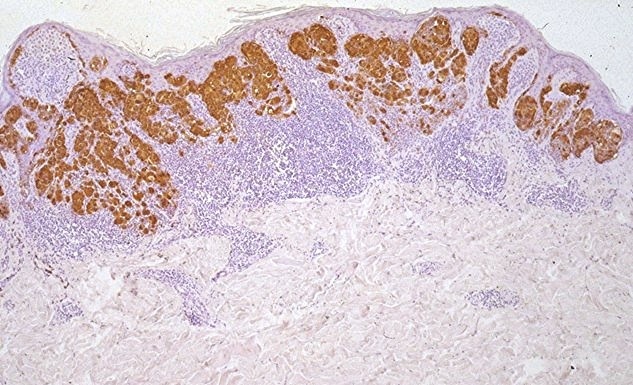
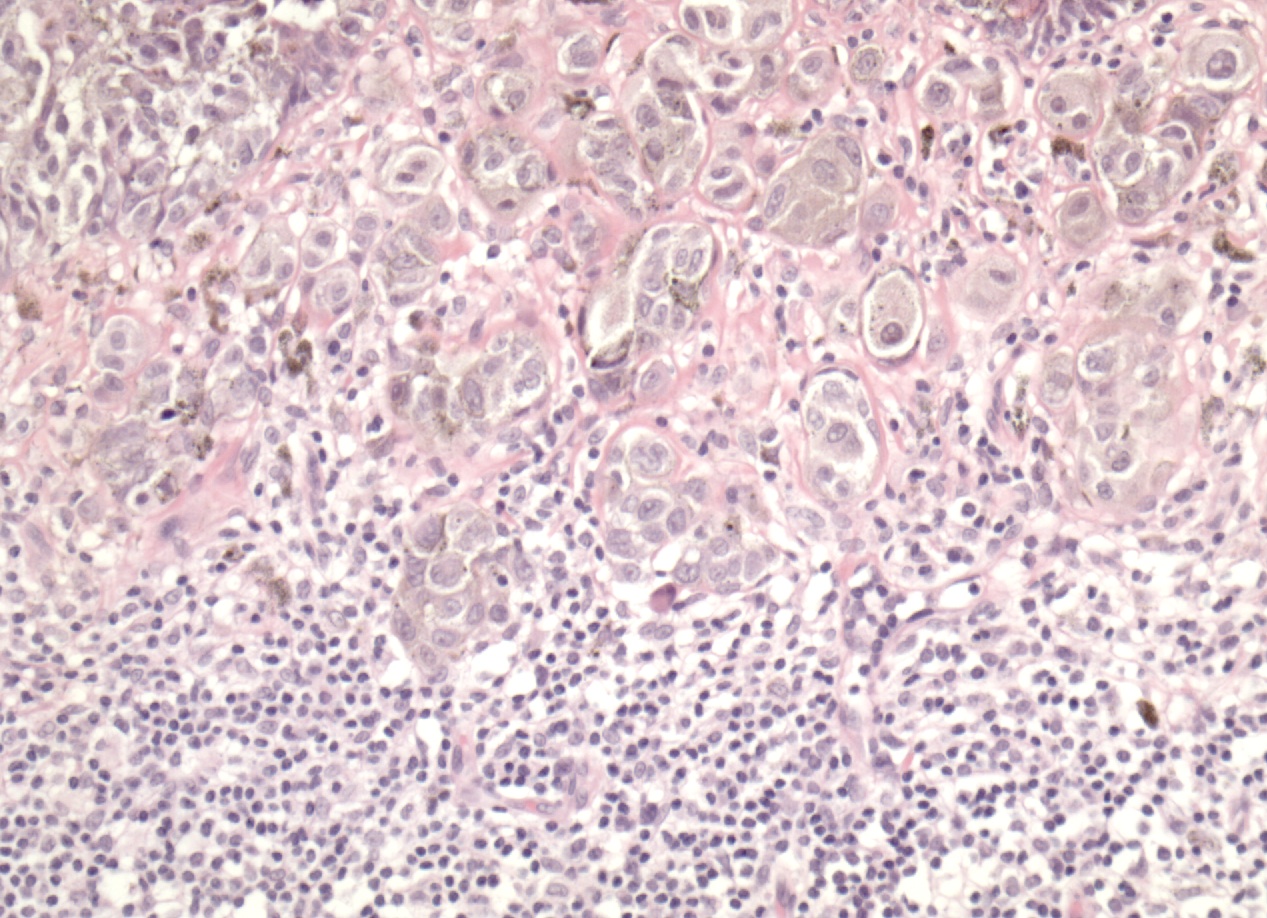
The prognostic role of tumor infiltrating lymphocytes (TIL) in patients with melanoma has been analyzed in several studies that yielded conflicting results. This might be related to the different methods used in evaluating and quantifying this parameter.
Despite the fact that the above mentioned clinicopathological factors are the most reliable prognosticators currently available, none of them or their combinations can accurately predict the clinical outcome on a single patient basis, as demonstrated by the relatively wide range of survival rates within each TNM stage (see TNM staging section).
Since the malignant phenotype is sustained by the complex molecular derangement of tumor cells, it is logical to expect that advancements in molecular oncology will provide clinicians with novel and more effective prognostic tools [8, 9].
Prognostic biomarkers are being sought mainly in the primary tumor by investigating the expression of molecules suggestive of cancer aggressiveness, which ultimately can be considered a surrogate marker of the risk for the patient of harboring minimal residual disease (MRD). As the molecular biology of melanoma is dissected, a growing number of molecular markers is being proposed. For some of them (e.g. Akt, MITF, PTEN, Bcl-2, NCOA3) a prognostic value has been claimed [10-14]. The discovery pace for novel prognosis indicators has been recently accelerated by the implementation of high-throughput technologies, such as gene microarray [15, 16]. However, the statistical independence of molecular markers from traditional clinicopathological factors (with particular regard to tumor thickness) has not been demonstrated in all series, which are often small and heterogeneous; moreover, results are rarely confirmed in different series.
The direct search for MRD is an emerging research field whose aim is to demonstrate the presence of microscopic melanoma residues not detectable by conventional imaging techniques or pathological examinations. Thus far, the only biomarker universally recognized as an useful prognostic factor is lactate-dehydrogenase (LDH): its plasma levels are in fact included in the TNM system for patients with distant metastatic disease (LDH is of no use for earlier disease stages) [1, 2]. Other single plasma proteins have been proposed (e.g. S100, melanoma inhibitory activity [MIA], TA90), but no consensus exists on their prognostic power [17, 18]. Examples of more recent developments in this field are the sentinel node ultrastaging by means of PCR based methods [19], the detection of circulating tumor cells or free DNA in the peripheral blood [20], and the serum protein profiling by means of proteomics technologies [21]. However, results are often controversial, and overall no conclusive evidence is yet available.
Besides molecular factors with strictly prognostic value, biomarkers predictive of response to therapy are another active and fundamental field of investigation to select patients who most benefit from a given anticancer agent, which ultimately would lead to increase the therapeutic index (i.e., the ratio between clinical benefit and toxicity) of available treatments [8, 22]. However, as regards melanoma, this research field is still in its infancy [9, 23].
Thus far, there is no critical mass of scientific evidence for any biomarker to be implemented in the routine clinical management of patients with melanoma. This observation urges investigators to systematically include the study of biological correlates while designing clinical trials [24, 25].